Category: SOIL SERIES
 |
 |
Soil is the most fundamental resource for the farmer, without which food and natural fibre cannot be produced. This article forms part of a series to highlight this resource. In this article, the exchange of cations and the base saturation percentage are discussed.
Cations are fundamental to plant nutrition, because a variety of essential elements are adsorbed by plants in the form of ions, specifically in the positively charged format (cations). These elements and compounds are retained in the soil through electrical attraction and are made available to plants through cation exchange. The greater the ability of a specific soil to retain cations and make them available to plants, the better the potential fertility of the soil will be.
Because the clays are mostly negatively charged, cations (elements and compounds with a positive charge) are mostly adsorbed. We therefore focus on cation adsorption and pay less attention to anion adsorption. Cations that are adsorbed by the clays can be displaced through exchange. For example, two hydrogen (H+) ions can displace one calcium (Ca2+) ion, should the hydrogen ion concentration in the soil solution be increased (Figure 1).
 |
| Figure 1: A schematic representation of Ca2+ in the clay complex, which is displaced by two H+ ions. Vervang asb. Figuur verskaf apart |
The opposite reaction may take place again when the Ca2+ concentration is increased. The force with which cations are adsorbed (should the concentrations be the same) takes place as follows:
Al3+ > Ca2+ > Mg2+ > K+ > Na+ > Li+
In this series, also known Lyotropic series, Al3+ has the largest valence and the smallest hydrated ion radius, and is therefore bound the strongest. Na+ and Li+ are monovalent, have a large hydrated ion radius and are therefore adsorbed the weakest. Na+ ions can displace Ca2+ or Al3+ ions, but the concentration thereof must be considerably higher that of Ca2+, for example, before it would be displaced. There is therefore a balance between the concentration of adsorbed cations and the concentration of the non-adsorbed cations in the soil solution. When the concentration of a certain cation, for example Ca2+, is increased in the soil solution, it will displace the cations that are less strongly bound, Na+ for example, via exchange. In all cases, one Ca2+ ion will displace two Na+ ions, so that the system remains electrically neutral.
The cation exchange capacity (CEC) of a soil is the sum of the exchangeable cations that a soil can adsorb and exchange. It is measured in centimole (cmole) positive charge per kilogram of soil, or cmolc kg-1 (1 cmolec kg-1 = 1 me 100 g-1 which is outdated). A soil with a CEC of 10 cmolec kg-1, for example, will therefore be able to adsorb 10 cmole monovalent cations such as K+ and / or Na+ per kg of soil, but only 5 cmole divalent cations such as Ca2+ and / or Mg2+. Therefore, one mole charge is equivalent to 1 mole monovalent cations (K+, Na+ or H+) or ½ mole divalent cations (Ca2+, Mg2+) or 1/3 mole trivalent cations (Al3+).
In all cases the negative charge on the soil colloids must be balanced by the positive charge originating from H+, K+, Na+, Ca2+, Mg2+, Al3+ or other cations such as ammonia (NH4+). For example, 10 cmole negative charges on the soil colloids must be balanced by 10 cmole positive charges from the cations. [A mole is a number, given by Avogadro’s constant: 6,02214086 x 1023]
In exchange reactions, 1 mole monovalent cations (H+, K+, Na+) are needed to exchange with a ½ mole divalent cations (Ca2+, Mg2+) or a 1/3 mole of trivalent cations (Al3+). The opposite reactions are also possible, for example: 1 mole K+ or 39 g K+ will be displaced by ½ mole Ca2+ or 40/2 = 20 g Ca2+; or 1 cmole K+ = 0,39 g K+ kg-1 can be displaced by 0,5 cmole Ca2+ = 0,20 g Ca2+. [The relationship between one mole of a substance and the mass thereof is given by the molecular mass in the periodic table.]
The CEC of a soil sample is chemically determined via a number of chemical exchange reactions in the laboratory. Firstly, all non-adsorbed salts are washed out of the soil. Then the exchangeable cations are leached out of the soils using NH4+-acetate. Finally, the concentration of the adsorbed NH4+ is determined. It is this concentration which is equated with the CEC.
The negative charge in soil originates from the clay, organic and amorphous colloids. The amount of the negative charge differs for the various colloids. The CEC of soils will therefore also differ according to the type of colloid and the amount thereof in the soil. The CEC will increase with an increase in the clay content and will vary according to the clay mineral composition. For example, take a soil with 30% clay, comprising 10% kaolinite, 10% fine mica and 10% montmorillonite. One kilogram of this soil will therefore contain 300 g clay, which is composed of 100 g of each of the three clay minerals. The negative charge is thus: 1 cmole for kaolinite (10/1000 X 100 = 1 cmolec 100 g-1), plus 3 cmolec for fine mica and 10 cmolec for montmorillonite. The CEC of the soil is therefore 14 cmolec kg-1 soil. Apart from the type and quantity of colloids in the soil, the CEC is also dependent on the pH of the soil, as a result of the pH dependent charge. The CEC of the soil will therefore increase should the pH of the soil increase after lime has been added, for example.
The CEC not only determines the potential fertility of the soil, but also restricts pollution of the ground water. Most of the plant nutritional substances are cations (Ca2+, Mg2+ & K+) which are adsorbed in the soil and are released for plant uptake. Soils with a CEC lower than 5 cmolec kg-1 tend to lose nutrients more easily through leaching than soils with a higher CEC. Soils with a CEC greater than 15 cmolec kg-1 are therefore regarded as naturally fertile because they are resistant to a decrease in nutrients through leaching and plant uptake.
Soil is not only a medium to be used for food production, but is also used to get rid of waste products. All positively charged waste products can be adsorbed in soils with a high enough CEC. In this way, pollution of ground water is restricted.
The adsorbed cations can be divided into acid cations (H+ en Al3+) and basic cations (Ca2+, Mg2+, K+, Na+, NH4+). The acid cations are non-essential plant nutritional materials and are therefore detrimental for plant nutrition because they occupy negative charges which should have been available to the basic cations (they also have an acidic reaction in the soil). The percentage made up by the basic cations on the exchange complex (base saturation percentage) is therefore and important parameter in the determination of soil fertility. The base saturation usually decreases as the soil pH decreases.
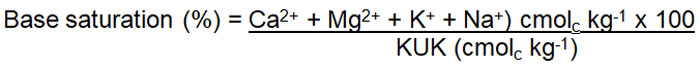 |
|
|
Apart from the base saturation, the concentration of each cation and the relationship they have with each other is also important in the determination of the fertility of the soil. The next article will discuss pH and the role it plays in soil.
In terms of plant nutrition, the availability of basic cations is extremely important. There are major differences between soils in terms of the amount of plant nutrients they can retain. This is expressed in terms of the soil’s CEC. The more the CEC is filled with these plant nutrients, the more the soil can be maintained according to its potential plant growth. It is therefore important to improve and maintain the soil’s fertility status over a period, so that it does not degrade.
For further information, please contact Martiens du Plessis at 072 285 5414 or martiens@nwk.co.za or Cornie van Huyssteen at 051 401 9247 or vanhuysteencw@ufs.ac.za.
The following sources have been used extensively during the compilation of this article: