Category: SOIL SERIES
 |
 |
Soil is the most fundamental resource for the farmer, without which food and natural fibre cannot be produced. This article forms part of a series to highlight this resource. In this article, we discuss the most important clay minerals occurring in the soil.
Primary minerals weather chemically to form the basic elements for the formation of secondary minerals. Because oxygen (O; 46,6%), silicon (Si; 27,7%), and aluminium (Al; 8,1%) are the most prolific elements, in the earth’s crust, one may conclude that these three elements will also be the most prolific in the secondary minerals.
The silicon cation is surrounded by four oxygen anions to form a four-sided tetrahedron. The tetrahedral clay sheet forms when the tetrahedra arrange themselves to form a layer. The aluminium cation is surrounded by six hydroxide (OH) anions to form an eight-sided octahedron. These octahedral also arrange themselves into a sheet, which is then referred to as the octahedral layer. It is combinations of these tetrahedral and octahedral layers that form the basic building blocks of the clay minerals. Because these secondary minerals occur mainly in the clay fraction and, as a result of the layered nature thereof, the terms secondary, clay or phyllosilicates (layered) minerals are used interchangeably, but they all refer to the same thing.
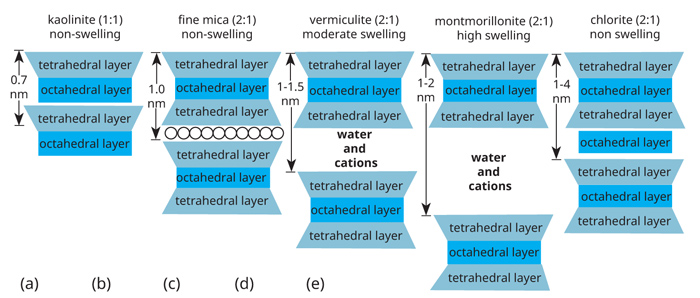 |
| Figure 1: Schematic representation of (a) a 1:1 clay mineral (kaolinite), (b) a non-expansive 2:1 clay mineral (fi ne mica), (c) a moderately expansive 2:1 clay mineral (vermiculite), (d) an expansive 2:1 clay mineral (montmorillonite) and (e) a non-expansive 2:1:1 clay mineral (chlorite). |
Kaolinite is the most common clay mineral in poorly structured soils, which include the vast majority of the arable land in South Africa. Kaolinite is formed when one Al octahedral layer and one Si tetrahedral layer combine to form a 1:1 clay mineral (Figure 1a and Figure 2). Limited isomorphic substitution (replaced by the same shape) of Al3+ which replaces Si4+ in the tetrahedral layer, leads to the formation of a negative charge of between 10 and 15 cmolc kg-1 of clay. [One mole is equal to 6,023 x 1023 (Avogadro’s number) particles. One centimole is therefore one hundredth, or 6,023 x 1021 of this. Therefore, kaolinite has 10 x 6,023 x 1021, or 6,023 x 1022 (60 230 000 000 000 000 000 000) negative charges per kg of clay.] This charge has to be balanced by cations and gives clay the ability to bind cations – the so-called cation exchange capacity (CEC). [Kaolinite’s charge is mainly pH dependent – more about this in a later issue.] Hydrogen bonding takes place between the Al-OH octahedral layer and the adjacent Si-O tetrahedral layer, of the two respective clay layers. This bond is relatively weak, but is strong enough to prevent water moving in between the layers. As a result, kaolinite minerals do not expand and shrink during wetting and drying. It therefore has stable physical properties. Kaolinite clay crystals are relatively large and occur in the coarse clay fraction. The clays are therefore non-sticky and are “non-plastic”. The kaolinite minerals occur towards the end of the weathering cycle, are rather resistant to weathering, and weather further to form aluminium oxides. As a result of the low CEC, it does not retain many cations and is therefore a poor provider of basic cations (potassium, calcium, magnesium and sodium). Halloysite is also a 1:1 clay mineral, but of lesser importance in soil.
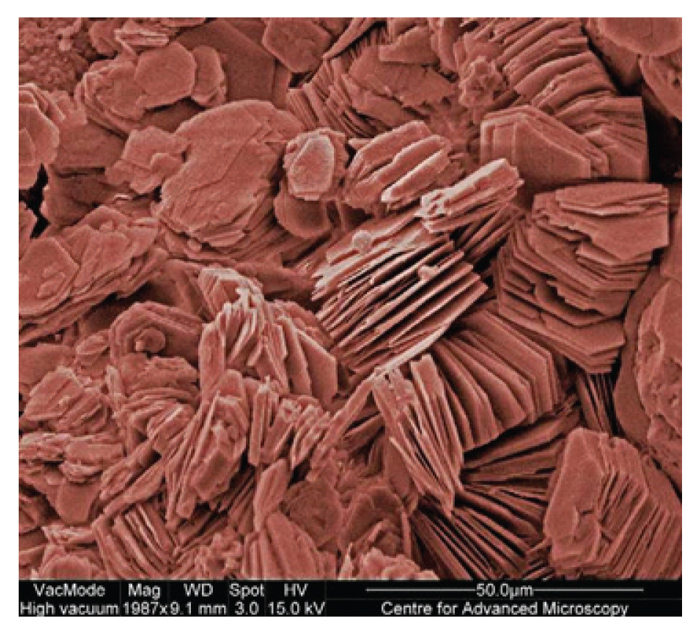 |
| Figure 2: Scanning electron microscopic image of kaolinite crystals, which indicates the hexagonal (six-sided) piles of crystals and interlayer spaces. (http://www.rdg.ac.uk/cfam/imageofthemonth/2008/February2008.html) |
The 2:1 clay minerals form when one Al octahedral layer occurs between two Si tetrahedral layers. The most important 2:1 clay minerals are the fine micas (Figure 1b), vermiculite (Figure 1c) and montmorillonite (Figure 1d).
The fine micas and vermiculite have undergone isomorphic substitution in the octahedral (Mg2+ replacing Al3+) and tetrahedral (Al3+ replacing Si4+) layers. In the case of the fine micas, the negative charge is balanced to a great extent by potassium (K+), which fits snugly between respective clay layers. As a result, the fine micas cannot expand and shrink during wetting and drying. The fine micas are an important source of K+ in the soil when it weathers. The nett CEC of fine mica is 40 cmolc kg-1 of clay.
Vermiculite forms when the fine mica minerals lose the K+ cations in the interlayer spaces due to weathering. Vermiculite clay, therefore, has a high CEC of 140 cmolc kg-1 of clay. Water and cations can move into the interlayer spaces but, because the clay strongly binds the cations, the clay has a limited expansion and shrinkage ability during wetting and drying. The most important property of vermiculite is that it can fix K+ in the interlayer spaces, making it inaccessible to plants. It is therefore a significant fixer of sink of potassium fertiliser in the soil, although this K+ can be released in the long-term through weathering.
Montmorillonite (Figure 1d and Figure 3) is also a 2:1 clay mineral, but has isomorphic substitution of Mg2+ which replaces Al3+ solely in the Al octahedral layer. The charge is therefore lower than is the case of vermiculite and is also concentrated in the middle of the clay mineral. The cations are therefore not attached as strongly as in the case of vermiculite and water, together with cations, can move easily in and out of the interlayer spaces. This results in montmorillonite expanding and shrinking considerably (almost doubling in size) during wetting and drying. These clay minerals are small and have a high CEC of 100 cmolc kg-1 of clay. The charges of 2:1 clay minerals are mainly permanent and are not influenced by pH. Montmorillonite clay minerals are very small and are therefore extremely sticky and “plastic” and have a high affinity for water and cations. These clays are therefore good providers of plant nutrients but, as such, can retain water so strongly that it is inaccessible to plants. As the soil shrinks when drying, it results in major cracks. Water infiltration will thus be rapid when the soil is dry, but very slow when wet. The sticky nature of the clay results in it being difficult to till when wet. When dry it is usually too hard to till. Soil comprising mainly montmorillonite is problematic to till and to irrigate, although it is very fertile. This soil is known colloquially as turf or black cotton clay.
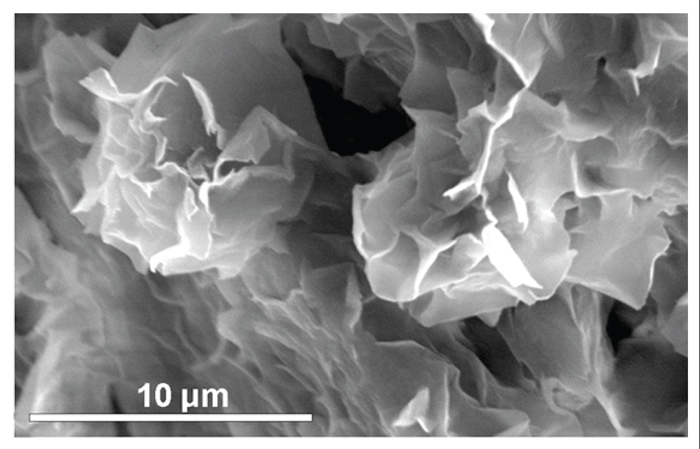 |
| Figure 3: Scanning electron microscope image of montmorillonite. The fineness and amorphous character of the mineral is clearly visible. (http://www.minersoc.org/pages/gallery/claypix/smectite/a-rose.html) |
Chlorite (Figure 1e) is a special 2:1 clay mineral, where an Mg octahedral layer is found in the interlayer spaces, which neutralises most of the negative charge. Chlorite is therefore in essence a 2:1:1 clay mineral. Isomorphic substitution, (Mg2+ replaces Al3+) in the Al octahedral layer, provides chlorite with a CEC of 40 cmolc kg-1 of clay. The properties of chlorite are very similar to those of the fine micas. They have a limited CEC and moderate hydration, expansion, and plasticity. The exception is that it is a good source of Mg2+ during weathering.
Clay minerals may be seen as the engine room of the soils, as it is the clay that retains water and cation plant nutrients. Although the various clay species comprise the same Al octahedral and Si tetrahedral layers, they have widely divergent properties, as a result of the varying degrees of isomorphic substitution in these layers. Knowledge of the type of clay mineral in the soil can therefore enable the land user to better manage their soils.
For further information, please contact Martiens du Plessis at 072 285 5414 or martiens@nwk.co.za or Cornie van Huyssteen at 051 401 9247 or vanhuysteencw@ufs.ac.za.
The following sources were used extensively during the compilation of this series of articles: