July 2025
| MARTIENS DU PLESSIS, SOIL SCIENTIST AND SOIL AND CROP CONSULTANT |
 |
IT IS ALREADY TIME TO START PREPARING THE FIELDS FOR THE FOLLOWING CROP SEASON. SOIL ANALYSIS IS ONE OF THE TOOLS IN THE FARMER’S ‘TOOLBOX’ TO MANAGE SOIL FERTILITY AND PLANT NUTRITION. IT IS THEREFORE WORTHWHILE TO TAKE A GOOD REPRESENTATIVE SOIL SAMPLE TO BE ANALYSED BY THE LABORATORY.
The first parameter in the soil analysis report is the soil’s pH. This article will provide some information on soil acidity, guidelines on liming and instructions on incorporating lime into the soil.
PURPOSE OF A SOIL SAMPLE
WHAT IS SOIL ACIDITY AND WHAT CAUSES IT?
Soil acidity is one of the main crop-limiting factors worldwide. Hydrogen and aluminium cations are largely responsible for soil acidity. The pH measurement indicates the hydrogen ions (H+) concentration in the soil solution. The pH of a neutral solution is 7.
The acidification of soil is both a natural and man-made phenomenon.
Natural acidification of soil
Carbon dioxide in the atmosphere dissolves in rainwater, causing the rainwater to become slightly acidic by forming carbonic acid. This carbonic acid dissolves calcium and magnesium in the soil, after which it leaches from the soil. Microbes and plant roots exude organic acids, which also leads to acidification. As plants take up calcium, magnesium and potassium from the soil, these cations are replaced by hydrogen and aluminium cations, which are acidic.
Man-induced acidification of soil
Emissions from the combustion of coal, as well as from fossil fuel combustion in engines, cause sulfuric and nitric acids to form in the atmosphere. These acids also dissolve in rainwater to form ‘acid rain’. This acid rain is a major cause of soil acidification in the Eastern Highveld. The next major cause of soil acidification is nitrogen fertilising, like urea, ammonium-nitrate and ammonium sulphate.
Why is soil acidity bad for crop growth?
As the soil becomes acidic, it is stripped of basic cations such as calcium, magnesium and potassium, which are macro plant nutrients. This implies that the soil becomes infertile. Aluminium cations also ‘come loose’ and dissolve in the soil water, which is the medium in which plant roots grow and from which the plants take up nutrients. The aluminium cations are toxic to growing plant roots (Photo 2) and soil acidity may limit plant growth substantially (Photo 1).
Furthermore, most of the plant nutrients become less soluble as the soil pH declines, which causes the soil to become less fertile. Others, such as manganese, may become toxic. The ideal soil pH for most grain crops is slightly acidic, between a pH of 6 and 7 (measured in pure water). Laboratories commonly do the pH measurements in a potassium chloride (KCl) solution, which results in the pH being approximately one unit lower (e.g. pH(water) 6 approximately equals pH(KCl) 5).

Soil acidity is extremely detrimental to crop growth (left), while the crop growth is normal in the limed area (right).

Aluminium cations are toxic to growing plant roots and may limit root growth substantially.
RECTIFYING SOIL ACIDITY
Crop fields should be sampled regularly (recommended every three years). The soil acidity must be rectified through liming.
Types of lime
Limestone is the most common material used to neutralise the soil pH. Calcitic lime or dolomitic lime is commonly available and used in South Africa. The lime is mined and ground to a very fine powdery product. Other types are calcium and/or magnesium oxide, or calcium and/or magnesium hydroxide.
Sources of calcium, magnesium and sulphur
Adding lime to the soil rectifies the pH, as well as replenish calcium and magnesium as plant nutrients. Gypsum mixed with lime is also available, which provides even more calcium, but also sulphur, another macro plant nutrient.
Amount of lime required
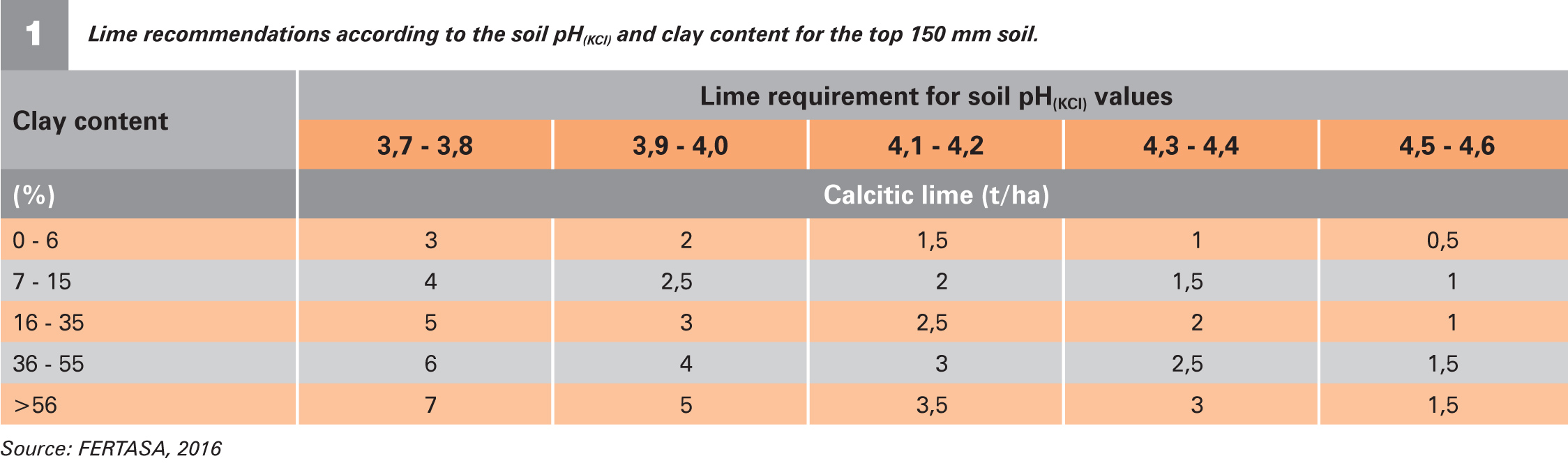
Generally, the higher the soil’s clay and humus content, the more lime is required to obtain the required pH shift. A pH shift is more easily obtained in sandy soils.
Table 1 shows the lime recommendations according to the Fertilizer Association of South Africa (FERTASA). These recommendations are sufficient to rectify the top 150 mm of the soil. When the sampling depth is 250 mm and the lime is ploughed 250 mm deep into the soil, the amount of lime must be increased accordingly. The sampling depth, lime requirement and depth of incorporation must be synchronised.
Fineness of lime
The time to complete the neutralisation reaction is directly correlated with the fineness of the lime. Very fine, powdery lime reacts much quicker than coarse particles. The powdery lime will react within a few months, given the soil is moist. The coarse particles will react over several months, even years. The fineness does not change the amount of lime needed. Even super-fine powdery lime induces the same pH shift as commercially available lime.
SPREADING OF LIME AND INCORPORATION INTO THE SOIL
Note: If the amount of lime was calculated for a soil depth of 250 mm, the lime must be incorporated and mixed with the top soil depth of 250 mm. The best way to mix and incorporate lime is by first discing and then ploughing. The better it is mixed with the soil, the better the reaction results will be.
CONCLUSION
Soil acidity is very common in our croplands and must be kept within the optimum limits to optimise the plant nutrition and eventually the crop yield. The importance of regular soil sampling and liming, according to the analysis results, cannot be overemphasised. Consult your local agronomist for the right amount and type of lime.
REFERENCES
Brady NC, 1990. The nature and properties of soils. Tenth Edition. Macmillan Publishing Company. New York
FERTASA, 2016. Fertiliser Manual. Eighth Revised Edition. FERTASA, PO Box 75510, Lynnwood Ridge, Pretoria
Publication: July 2025
Section: Pula/Imvula